Bent linear trigonal planar trigonal pyramidal. This would be T-shaped.
Chapter 8 Covalent Bonding 8 3 Bonding Theories Ppt Download
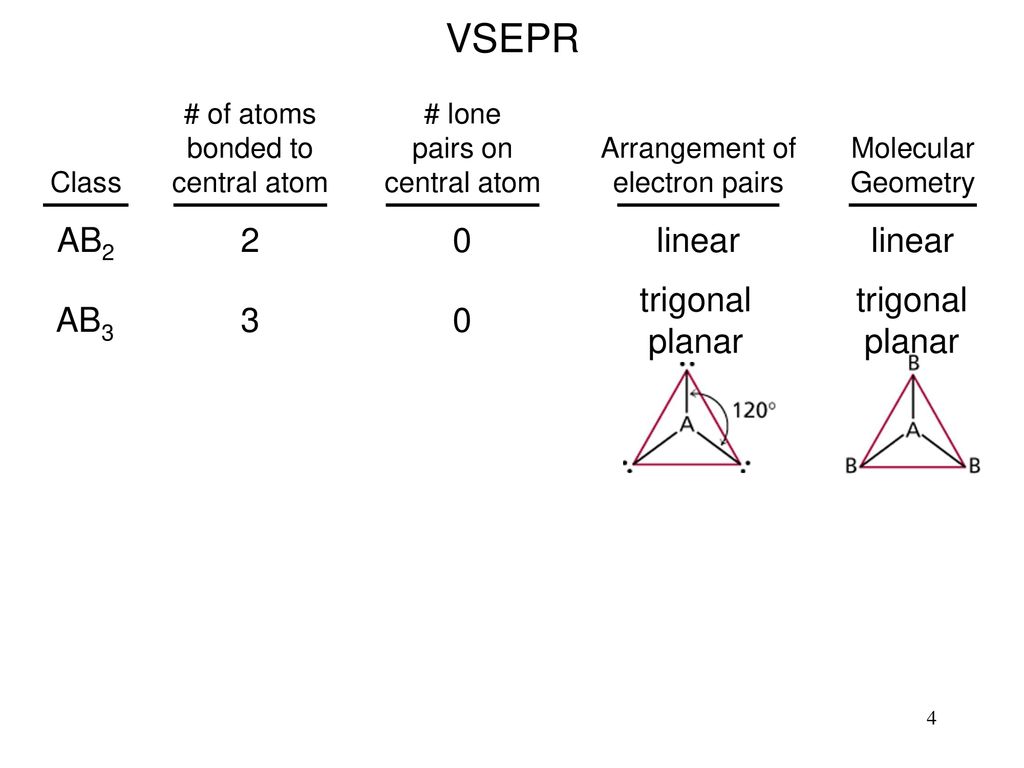
AX3-Forms a flat plane Ex.
. 3 types- dipole-dipole forces hydrogen bonding london-dispersion. A molecule with three atoms bonded to the central atom with one unshared pair of electrons. The forces of attraction between molecules are weaker than covalent and ionic bonds.
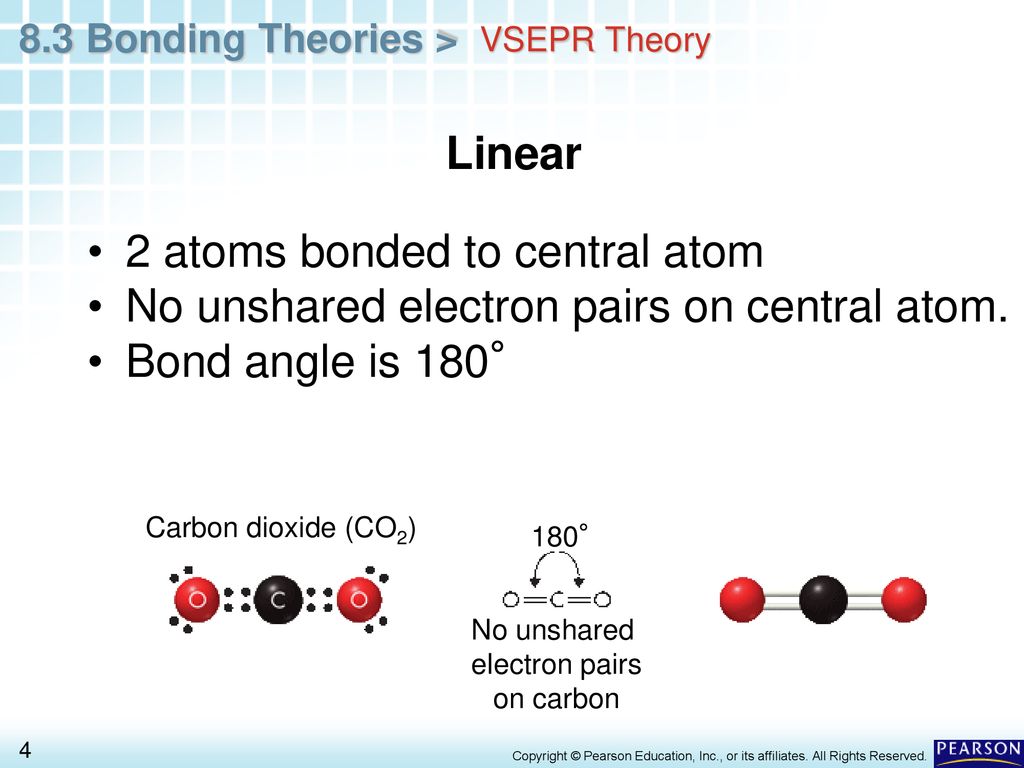
When there are two atoms bonded to the central atom and no lone pairs the molecule adopts a linear shape. A particle with two electron pairs and no lone sets encompassing the central atom has a linear shape. Again triangular bipyramidal assumes 5 atoms around the central atom.
As the attractions bring the atoms together electrons from each atom are attracted to the nucleus of both atoms which share the electrons. The shape of the molecule depends on the repulsion between the electron pairs present in the valence level. And the two bonds are arranged 180 from each other.
B A central atom with two lone pairs and two bonds to other atoms. 13 rows Groups is a more generic term. Assuming the central atom satisfies the.
Click to see full answer. Enter a value with a symbol. If two atoms are bonded to a central atom with no lone pairs how will they be arranged In a linear shape.
Therefore it can form four covalent bonds with other atoms or molecules. When lone pairs are. For example less than 90 degrees would be entered as.
The central atom of carbon dioxide is carbon which is doubly bonded to the two oxygen atoms. When lone pairs are present bent geometry can be present. What are the factors that determine the shape of the molecules.
What shape has 2 atoms bonded to the central atom and no lone pairs. What is the shape of the molecule. When there are two atoms bonded to a central atom with two sets of lone e-pairs what are the degrees of the resulting bond angle.
So including the electron pairs triangular bipyramidal is a good place to start. The force of attraction within molecules. A molecule has two bonded atoms around the central atom the central atom does not have any lone pairs what is the geometry of the molecules.
A molecule with a central atom and two bonded atoms is bent with a bond angle of 105. Trigonal Planar shape-The central atom is bonded to 3 other atoms-No lone pairs of electrons on the central atom-General formula. Group is used when a central atom has two terminal atoms.
Carbon has four such sharable electrons of its own so it tends to form four bonds to other atoms. How many bonds are in a carbon atom. When there are two atoms bonded to the central atom and no lone pairs the molecule adopts a linear shape.
When there are three atoms bonded to a central atom with no lone e-pairs are the degrees of the resulting bond angle. 2 lone pairs. Ignoring the lone pairs the molecular geometry is bent.
Input a one-word answer. It is tetravalent which means that it can form bonds. When there are four atoms bonded to a central atom with no lone.
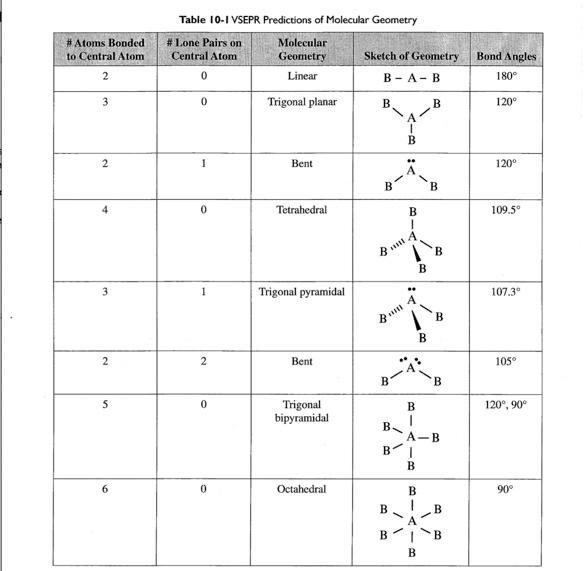
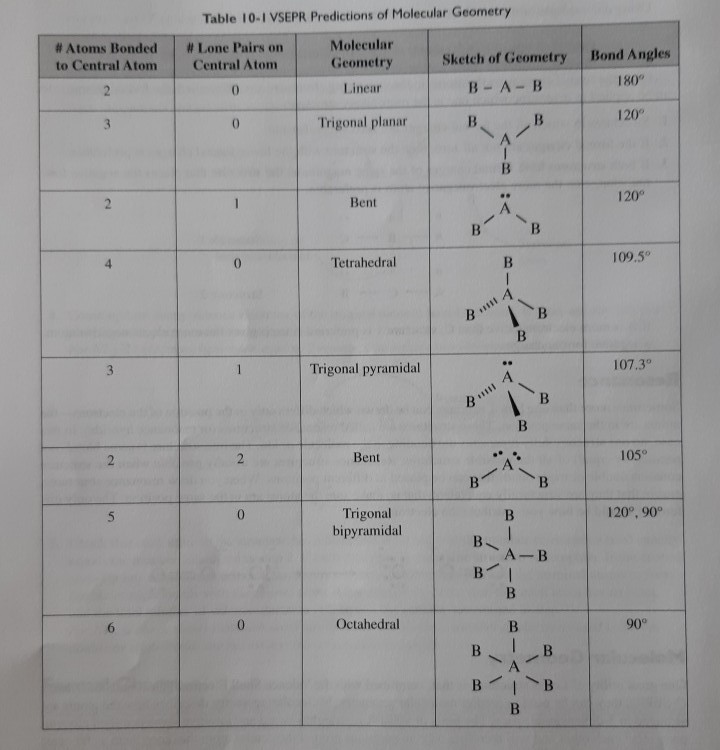
Refer to table above. Please see attachment for explanation. You might be.
Also asked what is the hybridization of the central atom in. A molecule with two atoms bonded to the central atom with ____ unshared paris of electrons has a linear shape. All have linear geometry.
In our case we have 3 atoms and 2 electron pairs. Sp hybridization occurs due to the mixing of one s and one p atomic orbital sp 2 hybridization is the mixing of one s and two p atomic orbitals and sp 3 hybridization is the mixing of one s and. The attraction of each atoms nucleus for the valence electrons of the other atom pulls the atoms together.
BF3 Dipole-None if the X3 are identical atoms-Yes if there are one or more different atoms depending on the EN 4. Carbon contains four electrons in its outer shell. The central atom has two outer atoms two bond pairs and two lone pairs.
When there are two atoms bonded to the central atom and no lone pairs the molecule adopts a linear shape. What is sp2 and sp3 hybridization. But since you only have 3 atoms 2 of the equatorial spots are occupied by the electron pairs.
A central atom. Hybridization12valency electron in central atomnoOf atom attached to central atom by single bondnegative charge-positive charge. One with four electron pairs and no lone pairs encompassing the central atom would have a tetrahedral shape.
A central atom with two lone pairs and three bonds to other atoms. In a typical bond two electrons are shared one from each of the atoms involved. The electrons surrounding the atom creates a 180-degree angle in the molecule which makes it form a linear molecular geometry.
C A central atom with two lone pairs and four bonds to other atoms. This would be bent eg water. Since electrons having the same.
Thus the hybridization state is sp3 s p 3 and the electron pair geometry is tetrahedral. Dvinal 7 10 months ago. This is clearly a water molecule in which two hydrogen atom is bonded to one oxygen atom.
A molecule with ____ atoms bonded to the central atom with zero unshared pairs of electrons has a trigonal planar shape. X atoms bonded to the central atom. When there are two atoms bonded to a central atom with no lone e-pairs what is the name of the shape of the molecule.
The valence electrons are involved in bonding one atom to another. When lone pairs are present bent geometry can be present. A molecule has two atoms that are bonded to the central atom and one lone pair of electrons around this central atom.
A central atom with four.
Solved V V Atoms Bonded To Central Atom 2 Table 10 1 Vsepr Chegg Com

Unit 2 3 Chemical Bonding Ppt Download
Solved 2 Using Table 1 Predict The Molecular Geometry Of Chegg Com
0 Comments